Pullan's Pieces #117- July 2016 Hottest targets for deals, biosimilars catch-22, CNS deals
Pullan's Pieces #117
July 2016
Global BD News and Analysis for Biotech and Pharma
Writing the newsletter always means a struggle to decide what to include. So many possibilities but I don't want to make a LONG newsletter. So I pick and choose. I'd be delighted to hear what you would like to see in the newsletter!
But I still want to give readers a UPDATE:
In May, I wrote in the case of "The Medicines Company v Hospira" which suggested that using a CMO might risk the patent, constituting a sale. Fortunately for many, the Court decided that CMO manufacturing does not constitute a sale before the patent application. http://www.fiercepharma.com/manufacturing/federal-court-rules-using-a-cmo-doesn-t-invalidate-drug-patents.
Hope you are enjoying the summer!
Linda
linda@pullanconsulting.com
1(805)-558-0361
www.PullanConsulting.com
Table of Content:
- CNS deals by indication and targets
- IPO market not bad
- Hottest targets for deals
- Biosimilars catch-22
CNS deals by indication and targets
Continuing from the CNS snapshot in last month's newsletter, I have analyzed CNS deals since 1/1/2011.
Alzheimer's Disease is the # indication in terms of number of deals, followed by Parkinson's and pain. Pain would take first place if the indication included chronic pain, osteoarthritic pain, migraine, acute pain and post-op pain Neurology is more common in deals than is psychiatry, with schizophrenia in 8th place in frequency of deals by indication.
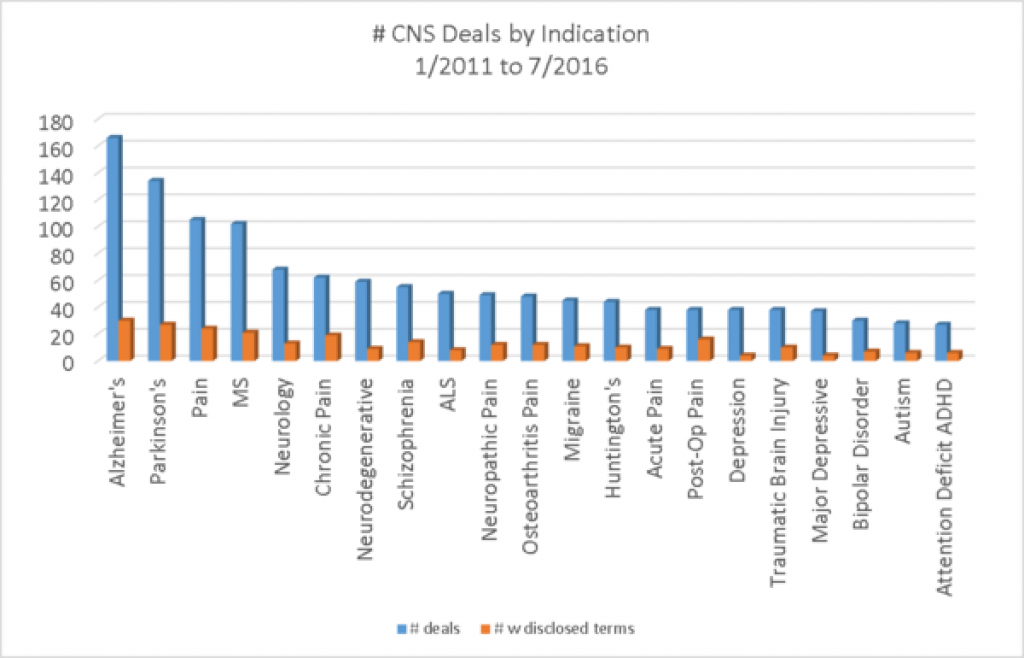
If we look at the number of CNS deals by target, pain targets make up the 3 most common targets and a few other positions as well. Beta amyloid, for Alzheimer's is the 14 target on the list, suggesting that there must be other diverse targets getting deals in Alzheimer's. Most of the targets are targets that have been worked on for years and years.
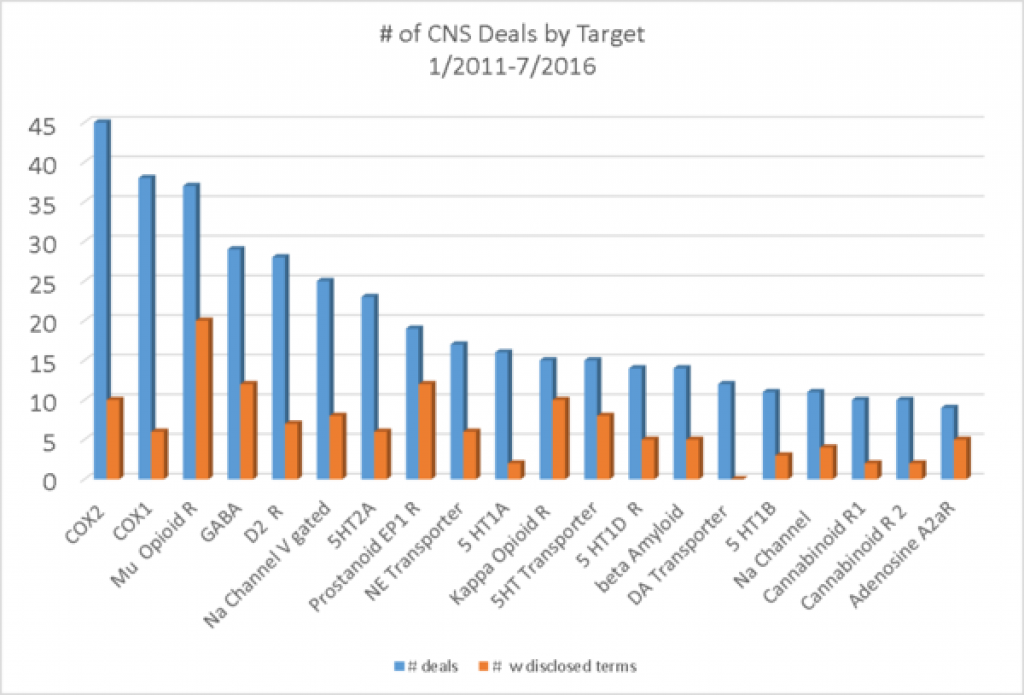
What if we look only at discovery or preclinical deals? This should tell us more about what is exciting in CNS drug discovery and indeed the list has a diverse set of targets.
Tau, the alternative target to beta amyloid for Alzheimer's is first in the frequency of deals. Adenosine 2a receptors and 5HT2a have been studied in many diseases. Nav1.7 is the sodium channel underlying inherited insensitivity to pain. HDAC2 is a regulator of gene expression. The Calcitonin receptor-like receptor is perhaps more commonly known as the CGRP receptor. Beta-secretase 1 is the processing enzyme for amyloid. Orexin receptors are implicated in sleep. The old favorite for pain, mu opioid receptors still makes the list for discovery and preclinical deals. I was surprised to see Activin receptor 2b on the list as I associate that with metabolism and muscle and bone, but apparently it is implicated in NMDA receptor signaling and LTP, underlying learning and memory.

If you would really like to hear more about "What's hot and what's not in CNS licensing", sign up for the upcoming free webinar sponsored by ShareVault. I will ask many questions of the experts!
IPO market not bad
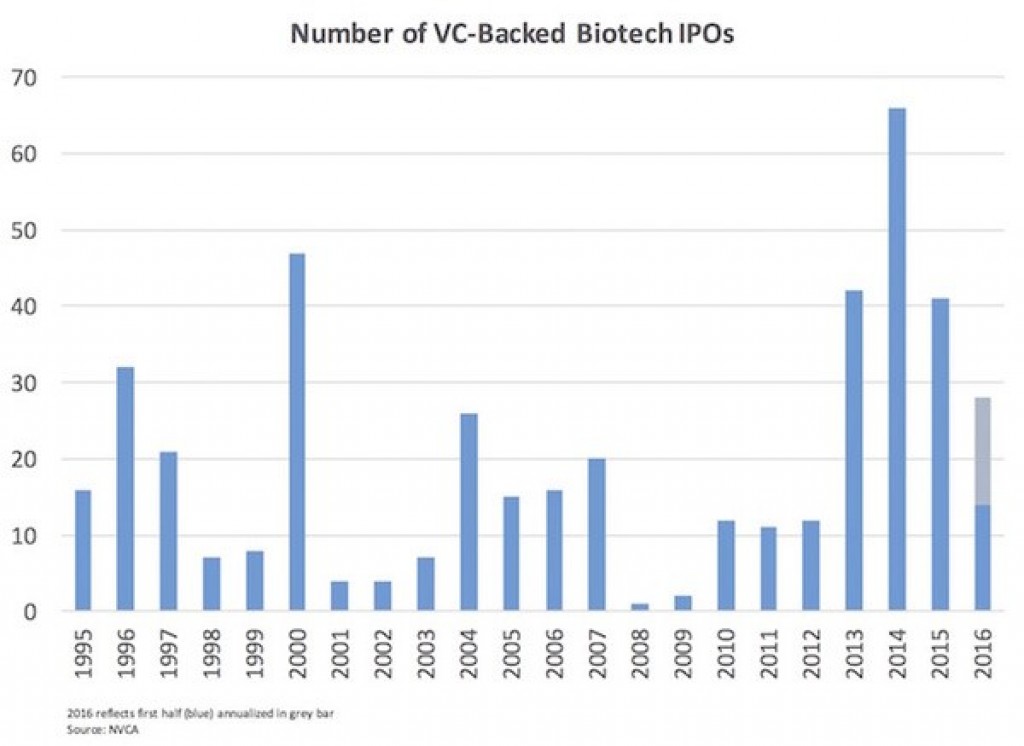
Bruce Booth reminds us that the IPO window is open.
Hottest target for deals in 2016?
Would you guess that PD1, the target of so much interest in immuno-oncology would be the subject of the most deals so far in 2016? That would have been my pick. But, that would be WRONG!
The hottest target for deals in 2016 is mu opioid receptor with 8 deals so far, to 7 for PD1. The mu opioid receptor comes in first over the last 5 and 1/2 years (since 1/2011) with 56 deals. COX2 and TNFalpha are tied for 2nd place with 53 deals each.
Biosimilars catch-22 - a fascinating FDA conundrum
Foley & Lardner's recent PharmaPatents blog highlights a catch-22 position for the FDA under the US Federal Circuit Court decision Amgen vs Apotex on the 180-day notice provision of the biosimilars provisions. The Court ruled that an applicant for FDA approval of a biosimilar must provide the originator 180 days notice before marketing can begin. The Court said this did not extend the 12-year biologics exclusivity of the originator by 180 days (6 months) because the FDA could give approval before the exclusivity runs out, to take effect when the exclusivity runs out. But the FDA has never given advance notice of an approval.
The FDA seems to have two bad options: Take a long time or get sued!
"Neither the Federal Food, Drug, and Cosmetic Act nor the Public Health Service Act explicitly grants the FDA authority to issue an approval that is effective at a later date. Rather, the statutes appear to be silent on this issue. Accordingly, it seems that the FDA would have to exercise one of two options to effectuate the court’s suggestion:
1. Initiate a long rule-making process to create a mechanism to grant pre-effective date approvals for biosimilar applications
2. Begin to grant pre-effective date approvals for biosimilar licenses without any regulations in place.
The first option would not be timely. The rule-making process likely would take years, and there is no guarantee that the final approval mechanism would resemble what the court envisioned.
The second option also is not realistic. If the FDA were to grant a pre-effective-dateapproval without regulations in place, it likely would be immediately sued by the original license holder. Additionally, the FDA historically has been uncomfortable taking any action that is inconsistent with FDA approval policies and procedures for other products."
https://www.pharmapatentsblog.com/2016/07/21/can-fda-implement-the-bpcia-as-the-cafc-suggested/
This will be fun to watch.